SIDE EFFECTS ASSOCIATED WITH ORAL CONTRACEPTIVES
Take the opportunity during the initial consultation and during check-ups at resupply to check if any of the conditions listed below have occurred while taking Lovima. It helps to talk about these medical conditions using terms the woman will understand and using signs and symptoms that she can more easily recognise. Although there is little evidence of an increased risk of these conditions listed below with Lovima or other POPs, it is important to recognise the symptoms.
SERIOUS SIDE EFFECTS ASSOCIATED WITH ORAL CONTRACEPTIVES
| Signs and symptoms | Possible condition | Action for patient |
|
Angioedema | STOP taking Lovima and seek immediate medical attention |
|
Thrombosis and blood clots (see 'common concerns with long-term use of oral contraception' section below for further information) | Seek immediate medical attention |
|
Liver disease (click here for further information) | Seek immediate medical attention |
|
Ectopic pregnancy. Note: The protection against ectopic pregnancies with traditional POPs is not as good as with COCs, as traditional POPs do not inhibit ovulation. The risk is considered to be lower with desogestrel compared with traditional POPs as ovulation is inhibited in up to 97% of cycles. |
Seek immediate medical attention |
|
Breast cancer (see below for further information) | Seek medical attention |
KNOWN SIDE EFFECTS OF DESOGESTREL (REPORTED)
The graphic below lists the possible side effects reported with regular use of desogestrel tablets such as Lovima, by system organ class and frequency.
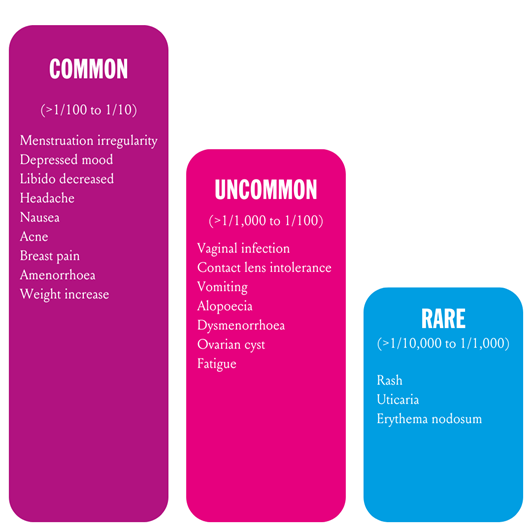
Several of the most commonly reported side effects are also reported with other POPs, including mood changes, altered bleeding patterns, decreased libido and weight changes.
BLEEDING CHANGES
The most commonly reported side effect is menstrual bleeding irregularity. Some kind of bleeding irregularity has been reported in up to 50% of women using desogestrel 75 microgram tablets. As Lovima results in closer to 100% ovulation inhibition in contrast to other POPs, irregular bleeding is more common than with other POPs. In 20-30% of the women, bleeding may become more frequent, whereas in another 20%, bleeding may become less frequent or totally absent. Vaginal bleeding may also be of longer duration.
After a couple of months of treatment, bleeding tends to become less frequent. Some women may find the change in bleeding frequency or duration, or the absence of bleeding an advantage. Women can be reassured that it is normal for their periods to become less frequent or stop altogether after a few months or treatment. The FSRH recommends that, as a guide, women considering desogestrel tablets such as Lovima can be advised that after 12 months of use, over a 3-month period approximately.
- 5 in 10 women can expect to have infrequent bleeding or no periods
- 4 in 10 women can expect to have regular spotting/ bleeding episodes (3-5 episodes)
- 1 in 10 women can expect frequent spotting/ bleeding episodes (>6 episodes)
- 2 in 10 women will experience a prolonged spotting/bleeding episode (lasting > 14 days).
COUNSELLING TIPS [2]:
- Inform women of the potential impact on their bleeding pattern.
- Irregular bleeding is not a sign that Lovima is not working
- In general, the woman does not need to take any action and can continue to take Lovima.
- However, if she is worried about changes in her menstruation or if bleeding becomes very frequent, irregular or heavy, she should be instructed to consult her doctor.
- Information, counselling and a bleeding diary can improve the patient's acceptance of changes in bleeding pattern.
Changes in mood
Depressed mood and depression have been reported as possible side effects of hormonal contraceptives. Depression can be serious and is a well-known risk factor for suicidal behaviour and suicide. Up to 1 in 10 women have reported depressed moos when using hormonal contraceptives, including desogestrel. However, there is no evidence of a causal link between the use of POPs and mood changes or depression.
COUNSELLING TIP:
Advise women that if they experience mood changes or depressive symptoms, they should contact their doctor for medical advice as soon as possible.
COMMON CONCERNS WITH LONG-TERM USE OF ORAL HORMONAL CONTRACEPTION
Does long-term use of the pill increase cardiovascular risk?
The evidence available to date does not support an association between POPs and the risk of cardiovascular disease and there is no evidence that they increase blood pressure. The benefits of using a POP are regarded as outweighing any theoretical or potential risks even when used by women with vascular disease.
COUNSELLING TIP:
Advise the women that there is no evidence that POPs increase blood pressure and the product can be used even by women with heart disease (aside from a blood clot).
'Does long-term use of the pill increase the risk of blood clots?'
The evidence available to date does not support an association between POPs and the risk of VTE. an association between the use of COCs and an increase in the incidence of VTE has been shown. The clinical relevance of this finding for oral contraceptives such as desogestrel, which lack an oestrogenic component, is unknown. However, it is still recommended that Lovima 75 microgram film-coated tablets should be discounted in the event of thrombosis and that women with a history of thromboembolic disorders should be made aware of the possibility of a recurrence. There are conflicting data for the risk of arterial embolic events (ATEs), such as myocardial infarction and ischaemic/cerebral stroke, among those using third-generation oral contraceptives including desogestrel. It is understood that the risk of stroke or myocardial infarction (MI) is comparable to second-generation oral contraceptives, where there is a very small, relative risk of ischaemic stroke in healthy women, particularly among older age groups.
More broadly, guidelines from the FSRH note that POPs have not been associated with an increased risk of stroke. However, as the risk of ATEs is increased among women who smoke, have diabetes, hypercholesterolaemia or hypertension pharmacists can play an active role in advising women on how to modify these risk factors.
COUNSELLING TIP:
- Counsel the woman on the need to seek IMMEDIATE medical advice in the event of a suspected thrombosis.
- Advise that she should return to the pharmacy or visit her GP if she is due to be immobilised or is to have surgery (ideally she should contact her doctor at least 4 weeks in advance).
'Does long-term use of the pill increase the risk of breast cancer?'
The risk of breast cancer increases in general with increasing age, and is also associated with a variety of factors including obesity and alcohol drinking. According to Cancer Research UK, less than 1% of breast cancer cases in the UK are caused by oral contraceptive use.
The risk in users of POPs, such as desogestrel, is thought to be of similar magnitude to that associated with COCs. Breast cancer has been found slightly more often in women who take COC pill, this reduces the risk, so that 10 years after stopping the COC pill, the risk is the same as for women who have never taken it. It is important to understand that breast cancer is rare in those under the age of 40, but that the risk increases as the woman gets older. As such, the extra number of breast caners diagnosed is higher if a woman continues to take the COC pill when she is older. The increased risk is not linked to the duration of use but to the age of the woman. In every 10,000 women who take the COC pill for up to 5 years but stop taking it:
- by the age of 20, there would be less than 1 extra case of breast cancer found up to 10 years after stopping, in addition to the 4 cases normally diagnosed in this age group;
- by the age of 30, there would be 5 extra cases in addition to the 44 cases normally diagnosed;
- by the age of 40, there would be 20 extra cases in addition to the 160 cases normally diagnosed.
Compared with the risk of getting breast cancer ever in life, the increased risk associated with COCs is low. The cases of breast cancer diagnosed in COC users may be due to an earlier diagnosis, biological effects of the pill or a combination of both.
COUNSELLING TIP:
Women should be reminded to check their breast regularly for lumps, nipple discharge and other breast symptoms as part of their normal health routine. Patients should be advised to contact their doctor as soon as possible if any such changes occur. Please visit https://www.nhs.uk/common-health-questions/lifestyle/how-should-i-check-my-breasts/ for further information. This is also an opportunity to remind women to take part in routine cervical screening.
