You will encounter many different scenarios concerning women visiting the pharmacy to ask about contraception and specifically Lovima. In all scenarios, you will need to check that the woman is (still) suitable for supply and that this is the right contraceptive option for her. Information on when to supply and how to take Lovima in different scenarios is provided in this chapter. Depending on the woman's scenarios, there may be additional areas to consider. You should investigate these areas to ensure you provide the women with the most appropriate advice.
Links to further information provided by the FSRH, Royal Pharmaceutical Society (RPS) and General Pharmaceutical Council (GPhC) on Sexual health consultations can be found in the 'signposting and further reading section'.
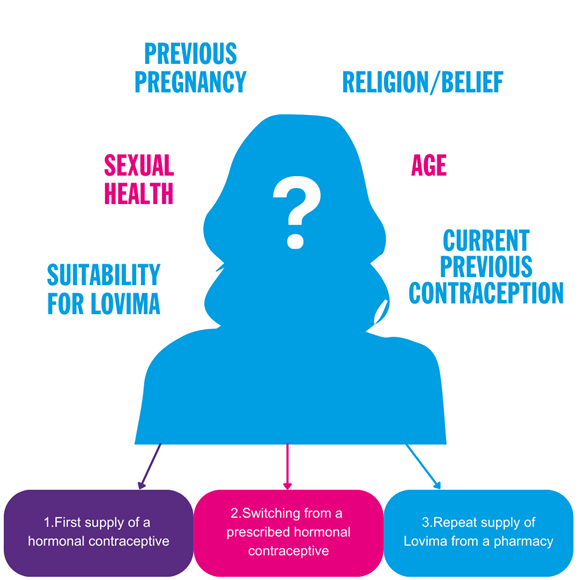
Fig 4 l Factors to consider when supplying Lovima
SUMMARY FOR SUPPLYING LOVIMA
STIs and sexual health consultation.
The consultation should be used as an opportunity to discuss general sexual health as well as contraceptive options, including LARCs so the patient can make an informed choice. Women should advised that Lovima 75 microgram film-coated tablets do not protect against HIV infection and other sexually transmitted diseases (STDs), and that they should use a barrier method in addition to their contraception if they have any concerns regarding STIs/STDs. Condoms could also be offered with the supply of Lovima if they are available and a risk is identified.
1.LOVIMA 75 MICROGRAM FILM-COATED TABLETS AS THE FIRST SUPPLY OF A HORMONAL CONTRACEPTIVE
There are various scenarios where Lovima may be considered to be the first supply of a hormonal contraceptive. The recommendations for how to take Lovima differ between these scenarios, and it is important that you provide the appropriate advise (see 'who can take Lovima' section). It is important to establish what contraception the woman is currently using (e.g. abstinence or a barrier method) and confirm that she is not pregnant. Consideration should be given to any safeguarding and consent issues with vulnerable women visiting the pharmacy (see section on 'age and safeguarding considerations').
2. SWITCHING FROM ANOTHER PRESCRIBED HORMONAL CONTRACEPTIVE TO LOVIMA
The type of hormonal contraceptive the patient was previously receiving impacts hoe they should take Lovima, and it is important that you provide the appropriate advice to the patient ( see 'who can take Lovima' section). You should check that she has been using the prescribed method correctly and therefore rule out any risk of pregnancy. It may be that a woman has lost or forgotten her prescribed contraceptive, and you should check whether you are able to provide an emergency supply of her current prescribed contraceptive rather than switch her to Lovima.
3.REPEAT SUPPLY OF LOVIMA 75 MICROGRAM FILM-COATED TABLETS FROM A PHARMACY
Some women asking for a supply of Lovima may have been using it previously and this is a repeat supply. At each request for a repeat supply, conduct a thorough follow-up consultation to ensure the woman is still suitable for Lovima, exclude pregnancy and ensure there have been no changes to the patient's health that impact the appropriateness of continued treatment.
Advise the woman that is she thinks she may be pregnant at any time when taking Lovima, she should confirm she is pregnant before stopping Lovima. The risk of foetal damage with Lovima is low. Stopping Lovima on the basis of a suspected pregnancy would leave the woman without contraception and potentially lead to an unplanned pregnancy. Ask if there have been any changes to her:



Take the opportunity during the check-ups at resupply to check if any of the conditions listed in the 'possible side effects' section have occurred while taking Lovima, and particular conditions that require immediate medical attention, such as VTE, liver problems and ectopic pregnancy (see the section on 'possible side effects'). The woman can find information in the PIL about health changes to which she needs to alert her doctor or pharmacist.
SUMMARY FOR SUPPLYING LOVIMA
Consider the woman's scenario when determining the quantity of Lovima to supply. It is recommended that:
- For the first supply of Lovima from a pharmacy, up to 3 months' supply (84 tablets) can be provided. This ensures that those starting treatment with Lovima does not receive Lovima for prolonged periods without a follow-up consultation.
- For repeat supply, up to 12 months (4x84 tablets) could be considered in women over 18 years.
- Supply to women under 18 years should be limited to 3 months to ensure a regular opportunity to assess safeguarding, compliance and counselling on sexual health.
